The plastic industry serves as the backbone of modern civilization, however, it unfortunately raises a lot of concerns regarding the materials and chemicals used in their production. This article seeks to explain the very controversies plastics are known for: the chemical enhancements and components that make plastics both useful and controversial.
Plastics not only dominate our lives but are a big part of our day-to-day activities, and with this in mind, this blog post will cover the basics, starting with the building blocks of all plastics. Additives include but are not limited to: stabilizers, plasticizers, and flame retardants. In addition, we will also discuss these chemicals in the context of the environmental consequences they pose or the greener substitutes developed to lessen those impacts. Expect to gain an insight into not only the science behind plastic production but also the consequences it brings to our lives through this article.
What are the main chemical elements used to make plastic?
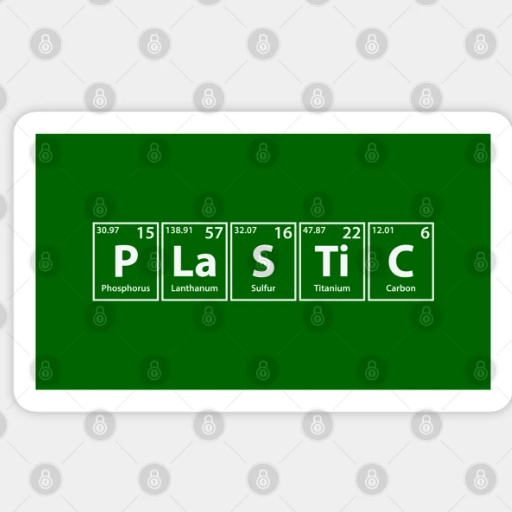
Plastics are primarily composed of carbon and hydrogen, with various other elements such as oxygen, nitrogen, chlorine, or sulfur incorporated depending on the type of plastic. These elements form the backbone of polymers, the long molecular chains that give plastics their versatile properties. Key building blocks include hydrocarbons derived from natural gas, oil, or coal, such as ethylene, propylene, and styrene. Additionally, additives like stabilizers and fillers are used to enhance the functionality and durability of the final products.
Carbon, hydrogen and other base elements in polymer chains
Carbon forms the backbone of most polymer chains due to its ability to create strong covalent bonds, providing stability and flexibility to the structure. Hydrogen is commonly bonded to carbon, forming hydrocarbons that serve as the primary components of many polymers. Additional elements, such as oxygen, nitrogen, chlorine, or sulfur, are frequently incorporated to modify the chemical and mechanical properties of the polymer. For instance, oxygen and nitrogen can introduce polarity, improving a polymer’s ability to interact with other substances, while chlorine often increases flame resistance.
The versatility of polymer properties arises from this diversity in base elements and their arrangements. Long chains of hydrocarbons such as polyethylene and polypropylene serve as foundational plastics, known for their durability and adaptability. Meanwhile, specific additives or variations in base elements can yield specialized polymers such as polystyrene (enhanced rigidity) or PVC (chlorine inclusion for chemical resistance). This intricate composition enables the creation of materials tailored to meet the demands of countless applications in industries such as packaging, construction, and medicine.
How crude oil and natural gas provide the building blocks for plastics
Crude oil and natural gas serve as the primary raw materials for producing plastics due to their rich hydrocarbon content. Through a process called cracking, these hydrocarbons are converted into smaller molecules like ethylene and propylene, which act as fundamental building blocks. These molecules are then chemically bonded through polymerization, forming long-chain polymers that constitute various types of plastic. Crude oil and natural gas, thus, provide the essential monomers—ethylene, propylene, and others—necessary for creating versatile plastics used across industries, from consumer goods to advanced technologies.
The role of ethylene, chlorine and other elements in different types of plastic
Ethylene is a key component in many plastics, such as polyethylene, which is widely used in packaging, containers, and pipes. Its simple chemical structure makes it highly versatile and easy to polymerize, producing materials with varying densities and strengths. Chlorine, on the other hand, is essential in the production of polyvinyl chloride (PVC), a durable plastic commonly utilized in construction, medical devices, and plumbing. By combining chlorine with ethylene, PVC gains properties like rigidity, flame resistance, and resilience to weathering. Other elements, such as oxygen or nitrogen, are often integrated into plastics like nylon or polyesters to enhance flexibility, strength, or thermal stability. These diverse chemical contributions enable tailored plastics to meet specific demands in industries ranging from everyday consumer goods to specialized engineering applications.
Which polymers form the foundation of plastic products?

The foundation of plastic products is primarily built on a range of key polymers. These include polyethylene (PE), which is widely used due to its versatility and durability, and polypropylene (PP), valued for its toughness and resistance to chemicals. Polyvinyl chloride (PVC) is another crucial polymer, known for its rigidity and weather resistance. Additionally, polystyrene (PS) is commonly used in packaging and insulation, while polyethylene terephthalate (PET) is essential in creating bottles and synthetic fibers. Each of these polymers forms the backbone of modern plastic manufacturing, catering to specific functional and industrial needs.
Understanding polyethylene, polypropylene, and polyvinyl chloride (PVC)
Polyethylene, polypropylene, and polyvinyl chloride (PVC) are versatile polymers with widespread applications due to their unique properties.
Polyethylene is the most commonly used plastic globally, distinguished by its high chemical resistance, flexibility, and durability. It comes in various forms, including high-density polyethylene (HDPE) used in containers and piping, and low-density polyethylene (LDPE), which is ideal for film applications like plastic bags and packaging.
Polypropylene stands out for its toughness and resistance to fatigue, making it suitable for products like reusable containers, automotive components, and textiles. Its high melting point makes it ideal for applications requiring heat resistance.
Polyvinyl Chloride (PVC) is known for its exceptional rigidity in its unplasticized form (uPVC), commonly used in construction materials like pipes, windows, and siding. Flexible PVC, obtained by adding plasticizers, is used in items like cables, flooring, and medical equipment. Its resistance to weather and chemicals makes it a preferred choice for long-lasting applications.
These three polymers are indispensable in modern manufacturing, serving a wide range of industries due to their tailored properties and adaptability. Their diverse functionality demonstrates the critical role plastics play in both industrial and everyday uses.
From monomer to polymer: how elements become linked together
When we talk about how elements link together to form polymers, it essentially comes down to a process called polymerization. From what I’ve researched, polymerization occurs in two main ways—addition polymerization and condensation polymerization. Addition polymerization involves monomers with double bonds that open up and connect in a chain-like fashion, without losing any atoms. On the other hand, condensation polymerization links monomers but also releases small molecules like water as a byproduct. These processes are crucial because they transform simple monomers, like ethylene or styrene, into complex and durable polymers, such as polyethylene or polystyrene, which we rely on in countless everyday applications.
Commodity plastics vs. specialty plastics: differences in composition
Commodity plastics are widely used, low-cost materials primarily composed of simple polymer structures, such as polyethylene (PE), polypropylene (PP), and polystyrene (PS). They are mass-produced for general-purpose applications like packaging, containers, and household items due to their ease of manufacture and cost efficiency. These plastics often have limited mechanical or thermal properties but are suitable for high-volume, low-stress uses.
Specialty plastics, on the other hand, are engineered for specific performance characteristics and high-demand applications. They are often composed of complex polymer structures or blends, such as polycarbonate (PC), polyetheretherketone (PEEK), and fluoropolymers. These materials exhibit superior properties, including enhanced strength, thermal resistance, chemical durability, and electrical insulation, making them essential for industries such as aerospace, automotive, and healthcare. While specialty plastics are more expensive and produced in smaller quantities, their advanced performance justifies their use in specialized and critical applications.
What chemical additives are commonly found in plastics?

Chemical additives are commonly introduced into plastics to enhance their properties and performance. These include plasticizers, which improve flexibility; stabilizers, protecting against heat and UV degradation; fillers, adding strength or reducing cost; and flame retardants, increasing fire resistance. Other additives like antioxidants prevent oxidation, while colorants provide pigmentation, and antistatic agents reduce static electricity on plastic surfaces. These additives are vital for tailoring plastics to meet specific industrial requirements.
Plasticizers, flame retardants, and stabilizers: their roles and compositions
Plasticizers are primarily used to increase the flexibility, durability, and workability of plastics. These compounds, such as phthalates or adipates, reduce the intermolecular forces in the polymer chains, allowing them to move more freely. They are commonly found in products like PVC, making it softer and easier to handle.
Flame retardants serve the critical function of reducing the flammability of plastics by either delaying or suppressing combustion. Popular types include halogenated flame retardants, organophosphorus compounds, and aluminum hydroxide. These additives operate by either chemically interfering with the combustion process or creating a protective barrier that shields the material from heat and oxygen.
Stabilizers are vital for protecting plastics from degradation caused by environmental factors like heat, UV radiation, or oxygen exposure. Heat stabilizers, such as metal soaps or organotin compounds, are used in applications like PVC to withstand high processing temperatures. UV stabilizers, such as hindered amine light stabilizers (HALS), shield plastics from sun damage, prolonging their useful life. These additives ensure materials maintain their mechanical and physical properties over time.
How additives in plastics affect the properties of the final product
Additives in plastics significantly influence the properties of the final product, tailoring it for specific applications or improving its performance. For instance, fillers like glass fibers or calcium carbonate can enhance strength, rigidity, and cost efficiency. Plasticizers increase flexibility and processability, making materials like PVC more versatile. Flame retardants, commonly used in electrical and construction applications, reduce flammability by interfering with combustion processes. On the other hand, color additives, including pigments and dyes, allow for aesthetic customization without compromising durability. Each additive works synergistically to refine characteristics such as durability, safety, and functionality, ensuring the material meets industry-specific requirements.
Potential concerns about chemical additives and human health
Chemical additives, while enhancing the functionality and safety of materials, have raised concerns regarding their potential health effects. Some additives, such as phthalates and bisphenols used in plastics, are suspected endocrine disruptors, interfering with hormonal balance. Flame retardants, particularly brominated types, have been linked to developmental and neurological issues due to their persistence in the environment and their accumulation in the human body. Additionally, prolonged exposure to certain color additives and preservatives has been associated with allergic reactions and other adverse effects. Though regulatory agencies impose strict limits on the use of harmful substances, ongoing research is crucial to assess long-term impacts and ensure human safety.
How do different elements affect the properties of plastics?

Various elements and additives incorporated into plastics significantly influence their properties, making them suitable for a wide range of applications. For instance, the addition of plasticizers enhances flexibility and durability, while stabilizers improve resistance to environmental factors like heat and UV radiation. Some polymers may include fillers to increase strength or reduce production costs. Colorants provide aesthetic versatility, and flame retardants improve safety by reducing flammability. Each element plays a specific role in tailoring the physical, chemical, or mechanical characteristics of plastics to meet diverse industrial and consumer needs.
Elements that influence durability, flexibility, and heat resistance
Durability, flexibility, and heat resistance in plastics are determined by a combination of material composition and additives. The polymer type used forms the foundation of these properties—for example, polycarbonate and polyethylene are known for their durability, while elastomers like silicone provide flexibility and heat resistance. Additives such as stabilizers protect plastics from degradation caused by heat and UV exposure, extending their lifespan. Plasticizers are key to enhancing flexibility, especially in materials like PVC, by reducing intermolecular forces and improving pliability. Heat resistance is often achieved by integrating flame retardants or cross-linking agents, which improve thermal stability and structural integrity under extreme temperatures. These elements are meticulously engineered to ensure plastics meet specific industry requirements for performance and reliability.
Materials such as cellulose and other derived from natural sources
Most industrial bioproducts are made from cellulose, also known as plant fibers. Cellulose is primarily gotten from the plant because it is renewable and can serve a variety of purposes. It is a component of the paper industry, textile industry including cotton and linen, and even in the bioplastics industry. Unlike synthetic plastics, which are extremely dangerous to the environment, cellulose can easily be broken down and does not pose any threats to the ecosystem. Furthermore, products like films, coatings and even some medicinal products can be made by using cellulose derivatives such as methylcellulose and cellulose acetate.
Other readily available natural sources include chitin, starch and lignin. Biodegradable materials like adhesives and packages can be made using starch, which is derived from crops like corn, potatoes and wheat. Chitin has an exoskeleton of crustaceans and is used in biomedical purposes as well as water purification due to the nature friendly attributes it possesses. Lignin is a byproduct obtained when making paper and is regarded as a potential source for aromatic chemicals as well as adhesives. All these materials obtained from mother nature plays an important role in decreasing dependence on fossil fuels and encourages further development in sustainable development.
The science behind biodegradable plastics and their elemental composition
Biodegradable plastics are designed to break down naturally through the actions of microorganisms such as bacteria and fungi, thereby reducing environmental impact compared to traditional plastics. These materials typically consist of polymers derived from renewable resources like starch, cellulose, or polylactic acid (PLA), as well as biocompatible petrochemical compounds. The decomposition process involves microorganisms metabolizing the polymer chains into simpler, organic compounds like water, carbon dioxide, and biomass under appropriate conditions of temperature, humidity, and oxygen.
Biodegradable plastics are primarily composed of elements such as carbon (C), hydrogen (H), and oxygen (O), forming the backbone of the polymers. Some variants may also include nitrogen (N) or other elements, depending on their formulation or intended use. Their structural composition allows for easier integration into the natural carbon cycle compared to conventional plastics, making them vital for mitigating plastic waste. However, biodegradability is highly dependent on environmental conditions, making proper disposal essential for their breakdown.
Which elements are problematic for plastic recycling?

Certain elements can complicate plastic recycling due to their impact on the recycling process or the quality of the recycled material. Contaminants like dyes, metals, and additives used to improve plastic functionality often hinder efficient recycling. For example, non-recyclable elements, such as certain pigments or flame retardants, can alter the chemical composition and reduce the purity of the recycled product. Additionally, materials like multi-layer plastics, which incorporate different types of polymers or include metal layers, are particularly challenging to recycle, as separating the components is complex and resource-intensive.
Challenges in sorting and processing different polymer types
Sorting and processing different polymer types can be quite complicated due to the diverse properties of plastics. From my research, I found that one major challenge is accurately identifying and separating polymers. Many plastics look similar, but slight differences in their composition can impact recycling effectiveness. Another issue is contamination—when plastics are mixed or contain residues, it becomes harder to achieve high-quality recycling results. Lastly, certain polymers require unique processing methods or temperatures, making it costly and technically challenging to recycle them efficiently. These complexities emphasize the need for innovations in recycling technologies and better material design.
How additives complicate plastic waste management and disposal
Additives for plastics, including plasticizers, stabilizers, flame retardants, and colorants, have become common in the industry because of their ability to enhance the performance and durability of plastics. Even so, the chemicals complicate recycling and disposal much more than necessary. Firstly, these additives may change properties of the polymer, which lessens the recyclability and increases the difficulty in producing consistent, high-quality uniform recycled materials. For example, mixed additives incorporated into a plastic may result in inconsistent melting points or structural integrity during processing. Secondly, some additives are dangerous because they emit hazardous materials when burned in waste or recycled, creating potential dangers for the environment and human health. Finally, the large number of these types of additives makes locating and determining the presence of their additives in a particular waste stream much more complicated, increasing the costs and time associated with processing and recycling plastic waste. These obstacles point to the need for further research on other types of additives and for improved labeling and identification systems within waste management.
The future of plastic recycling: designing with end-of-life in mind
From my perspective, the future of plastic recycling requires a proactive and innovative approach. First, addressing the challenge of mixed additives involves designing plastics with fewer, standardized components that are easier to recycle. Additionally, the development of biodegradable or chemically recyclable materials can reduce the impact of hazardous additives. Second, investing in advanced sorting technologies, such as AI-driven systems or improved chemical markers, will streamline the identification and separation of materials in waste streams. Lastly, collaboration across industries to implement clear labeling systems and promote circular design principles will ensure that products are created with their recycling potential in mind. By tackling these challenges head-on, we can significantly improve the efficiency, safety, and environmental impact of plastic recycling.
Where do the elements in plastic come from?
Plastics are primarily derived from hydrocarbons found in fossil fuels, such as crude oil and natural gas. These materials provide the essential elements—carbon and hydrogen—that form the backbone of polymer chains. Additionally, various additives, such as chlorine, nitrogen, oxygen, or sulfur, may be incorporated to enhance specific properties like flexibility, durability, or resistance to heat. These elements are sourced from both natural and synthetic compounds during the production and processing of plastics. Thus, the elements in plastic are a combination of fossil-fuel-based resources and chemically engineered additives.
From fossil fuels to plastic: the journey of carbon-based elements
The transformation of carbon-based elements from fossil fuels into plastic involves several intricate steps. First, crude oil or natural gas undergoes extraction and refinement, yielding key components like ethane, propane, and naphtha. These are subsequently processed in facilities called crackers, where high temperatures break the molecules into simpler hydrocarbons, such as ethylene and propylene. These small molecules are then chemically bonded through polymerization into long chains, forming polymers—the fundamental building blocks of plastics. Additional chemical treatments and the incorporation of additives further enhance desirable traits such as flexibility, durability, and strength. This complex yet highly efficient process enables the conversion of raw fossil resources into versatile materials that meet a wide range of industrial and consumer needs.
Alternative sources for plastics production beyond petroleum
The development of alternative sources for plastics has become an essential focus in reducing dependence on petroleum-based production. One promising avenue is bioplastics, which are derived from renewable resources such as corn, sugarcane, and cassava. These materials use naturally occurring polymers like polylactic acid (PLA) and polyhydroxyalkanoates (PHA), offering compostable and biodegradable options for many applications.
Another alternative lies in recycling waste materials to create new plastics. Chemical recycling techniques break down plastic waste into its molecular building blocks, enabling the production of new polymers without relying on virgin petroleum. This approach not only reduces the need for raw fossil fuels but also addresses plastic waste management challenges.
Additionally, advancements in algae-based plastics show great potential. Algae can produce bio-oils that serve as feedstock for polymer production, offering a sustainable solution with a lower environmental impact. These alternatives highlight the innovative progress in creating sustainable plastics while addressing global environmental concerns.
The environmental impact of extracting elements used in plastics
The extraction of raw materials used in plastic production, such as petroleum, natural gas, and even minerals like silica, significantly impacts the environment. Drilling and mining operations contribute to habitat destruction, soil erosion, and water contamination. These activities often release greenhouse gases, exacerbating climate change. Additionally, the high energy consumption during resource extraction intensifies the carbon footprint of plastic production. The environmental cost is further amplified by the potential for oil spills and other industrial accidents, which cause long-term ecological harm. To mitigate these effects, sustainable practices like reducing dependence on virgin resources and investing in alternatives such as recycled content and bio-based materials are critical.
References
Frequently Asked Questions (FAQ)
Q: What are plastic additives and why are they used?
A: Plastic additives are substances added to plastic materials to enhance their performance, durability, and appearance. They are used to produce plastics with specific properties, such as increased flexibility, color, or resistance to UV light.
Q: How do chemical additives affect the structure and properties of plastics?
A: Chemical additives can alter the structure and properties of plastics by modifying their chemical bonds, which can enhance or change their flexibility, strength, and durability, making them suitable for a wide range of products.
Q: What are some common elements used to manufacture plastics?
A: Plastics are primarily made from organic materials, and the production of plastics often involves elements like carbon, hydrogen, oxygen, nitrogen, chlorine, and sulfur, which are combined during polymerisation to create various types of plastics.
Q: How is plastic litter impacting the environment?
A: Plastic litter, including waste plastics like plastic bottles and plastic film, can have significant negative impacts on health and the environment. It contributes to pollution, harms wildlife, and is difficult to manage due to its persistence in the environment.
Q: What are some types of plastic used in food packaging?
A: Plastics used in food packaging include polyethylene, polypropylene, and polyethylene terephthalate, which are chosen for their strength, flexibility, and ability to protect food from contamination.
Q: How are plastics derived from monomers?
A: Plastics are derived from monomers through a process called polymerisation, where monomers are chemically bonded to form long chains, creating the polymer structure that makes up plastic materials.
Q: What role do engineering plastics play in manufacturing?
A: Engineering plastics are used to manufacture products that require high performance, such as automotive parts and electronic components, due to their superior mechanical properties and heat resistance.
Q: How does the disposal and recycling of plastics work?
A: The disposal and recycling of plastics involve collecting plastic waste, sorting it by type, and processing it into new materials. This helps reduce plastic debris in the environment and supports sustainability by enabling the recycling of plastics into new products.
Q: What is the significance of plastic packaging in everyday use?
A: Plastic packaging plays a crucial role in everyday use by protecting goods, extending shelf life, and providing convenience in the form of packaging film and plastic containers, contributing to the efficiency of product distribution and storage.



