Plastics are ubiquitous in our modern world, appearing in countless forms, shapes, and applications. Yet, there’s often uncertainty or confusion about what plastics truly are. Are they merely synthetic products of industrial design, or do they share a deeper scientific connection with natural substances? At the heart of this question lies a fascinating exploration of polymers—long chains of molecules that form the building blocks of both synthetic plastics and many materials found in nature.
This article aims to uncover the relationship between plastics and polymers, demystifying the science behind these essential materials. We’ll take a closer look at the definition of polymers, how they serve as the foundation of plastics, and what distinguishes artificial polymers from their natural counterparts. By better understanding these connections, we can appreciate not only the versatility of plastics but also the implications of their widespread use for technology, sustainability, and the environment. Whether you are a curious learner or someone seeking industry insights, this comprehensive guide will serve as a starting point to unravel the intricate link between plastics and polymers.
What exactly are polymers and how do they relate to plastics?

Polymers are large molecules made up of repeating structural units, typically bonded together in long chains. These can be naturally occurring, like cellulose and proteins, or synthetic, like nylon and polyethylene. Plastics, on the other hand, are a subset of synthetic polymers designed for versatility in applications. Essentially, plastics are polymers that have been processed and formulated with additives to achieve specific properties such as flexibility, durability, or resistance to heat. This close relationship means that all plastics are polymers, but not all polymers are plastics.
Defining polymers: long chains of repeating molecular units
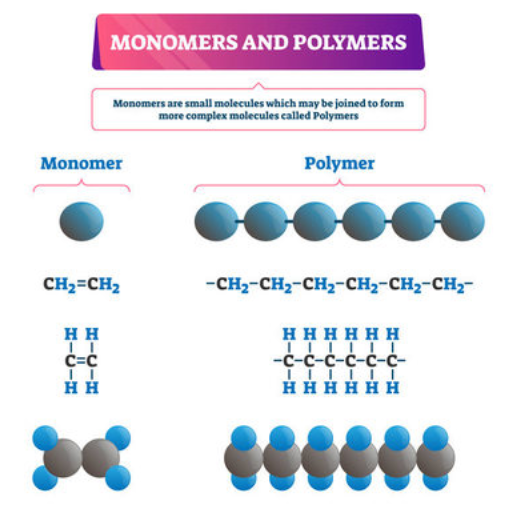
Polymers are considered as long chain macromolecules composed of repeating structural units called monomers. Depending on their chemical structure and length, these meric chains exhibit various properties and applications. The meric chains may occur naturally, such as cellulose and rubber, or they may be synthesized, like polystyrene and PVC. The overwhelming diversity of these polymeric materials and their remarkable characteristics such as strength, flexibility, and chemical stability is determined by the monomers and their configuration. The beneficial properties of these strikingly versatile polymers has led to their incorporation into products by various industries including textiles, construction, healthcare, and packaging.
The relationship between polymers and plastics: are all plastics polymers?
Plastics are a subset of polymers, meaning all plastics are polymers, but not all polymers are plastics. Polymers are large molecules composed of repeating structural units called monomers, and they can be naturally occurring, like silk or DNA, or synthetic. Plastics, on the other hand, are synthetic polymers specifically engineered for their versatility, moldability, and a wide range of applications. The defining feature of plastics is the addition of specific additives during their production, which enhance properties like durability, flexibility, or resistance to heat. Therefore, while all plastics belong to the broader category of polymers, many polymers, such as proteins and natural rubber, exist beyond the realm of plastics.
Natural polymers vs synthetic polymers: key differences
Natural polymers are substances derived from living organisms, such as plants, animals, or microorganisms. Examples include cellulose, proteins, and DNA. These polymers are typically biodegradable, eco-friendly, and play essential roles in biological systems. They are found abundantly in nature and are primarily formed through natural processes like polymerization in living cells.
Synthetic polymers, on the other hand, are man-made materials produced through chemical processes in industrial settings. They include commonly used materials like polyethylene, nylon, and polystyrene. Synthetic polymers are valued for their consistency, durability, and ability to be tailored for specific applications, but they tend to be less biodegradable and raise environmental concerns due to their persistence in ecosystems.
Key differences include their origin—natural polymers are biologically based while synthetic polymers are chemically engineered—and their environmental impact. While natural polymers are often renewable and decompose readily, synthetic polymers offer controllable properties but pose challenges in waste management and sustainability.
How are different types of plastic made from polymers?

Different types of plastic are made from polymers through processes that modify their structure and properties to suit specific applications. The process typically begins with monomers—small molecules derived from natural gas or crude oil—that are chemically bonded to form polymers through polymerization. Additives such as stabilizers, plasticizers, and colorants are then introduced to enhance durability, flexibility, or appearance. Thermoplastics, like polyethylene and polypropylene, are shaped by heating and cooling, while thermosetting plastics, like epoxy and phenolic resin, solidify permanently after curing. These processes enable a wide variety of plastic types, each tailored for unique functional requirements.
The polymerization process: how monomers link together
When small molecules, known as monomers, join together through a chemical process called Polymerization, long chain-like structures called Polymers are formed. Addition and condensation polymerization are the two primary types of polymerization processes. In addition polymerization, molecules known as monomers with double bonds, for example Ethene, combine without losing any small molecules. Condensation polymerization, on the other hand, occurs when monomers fuse together with the loss of byproducts like water or methanol. In any method of forming polymers from various monomers and conditions, chemical bonds are always broken and reformed. Furthermore, the conditions of the process also dictate the properties of the resulting polymer.
Hydrocarbon molecules: the building blocks of most plastic
Hydrocarbon molecules are crucial because they form the foundation of most plastics. These molecules, primarily derived from fossil fuels like crude oil and natural gas, consist of hydrogen and carbon atoms bound together. Their versatile structure allows them to be manipulated into long chains or polymers, which give plastics their strength, flexibility, and durability. By refining and modifying hydrocarbons through processes like cracking and polymerization, we can create a wide range of plastic materials tailored for different applications, from packaging to construction.
Types of polymer chains in different plastic materials
Polymer chains in plastic materials fall into three primary categories based on their structure and behavior:
Linear Polymers
Linear polymers consist of long, straight chains of repeating units. These chains are unbranched and tightly packed, contributing to high tensile strength and melting points. Examples include polyethylene (used in plastic bags) and polyvinyl chloride (PVC).
Branched Polymers
These polymers have side chains attached to the main chain. The branching reduces the packing efficiency and density, often leading to more flexible and lower melting point materials. A common example is low-density polyethylene (LDPE), used in lightweight packaging materials like plastic wraps.
Crosslinked Polymers
Crosslinked polymers have molecular chains connected through chemical bonds, creating a 3D network. This structure provides high rigidity, heat resistance, and durability. Examples include thermosetting plastics like epoxy resin and melamine, commonly used for coatings and robust, heat-resistant products.
These structural characteristics are key to tailoring plastic materials for specific uses, ensuring they meet the desired balance of flexibility, strength, or thermal and chemical resistance.
What are the main types of polymers used in plastic production?

The two principal categories in which polymers are classified with regards to their use in plastics are thermoplastics and thermosetting plastics.
Thermoplastics are reusable polymers that can be softened when heated to a certain temperature and hardened when cooled to another temperature. Polyethylene is a good example because it is used in packing while polypropylene is used in containers of various sorts. Further examples of thermoplastics include PVC (polyvinyl chloride) used in pipes and cables.
Thermoplastics are considered less damaging as they can be reused multiple times. The other category of thermosetting plastics are considered more damaging as they undergo a chemical change, resulting in the creation of an inflexible structure which does not soften upon application of heat. Epoxy resin, commonly used in adhesives and coatings, and melamine that is used for heat resistant dinnerware are examples of thermosetting plastics.
These are the basic building blocks of aside from the more complex materials, as these polymers allow for the creation of flexible and versatile forms of plastics that can be used across numerous sectors.
Thermoplastics: polyethylene, polypropylene, and PVC explained
Thermoplastics are versatile materials that become pliable or moldable above a certain temperature and solidify upon cooling, making them widely useful in manufacturing and engineering. Here’s a concise breakdown of three common types:
Polyethylene (PE): Polyethylene is the most commonly used thermoplastic, known for its toughness, flexibility, and chemical resistance. It is categorized into different types, like Low-Density Polyethylene (LDPE), used in plastic bags and film, and High-Density Polyethylene (HDPE), found in bottles and piping. Its lightweight nature and durability make it an ideal material for a variety of applications.
Polypropylene (PP): Polypropylene is valued for its high melting point, resistance to chemicals, and fatigue resistance. This thermoplastic is widely used in everyday products such as food containers, automotive parts, textiles, and medical equipment. Its ability to be easily molded and its structural integrity even after repeated bending are key advantages.
Polyvinyl Chloride (PVC): PVC stands out for its rigidity and versatility; it can be produced in both rigid and flexible forms. Rigid PVC is used in construction for pipes, window frames, and flooring, while flexible PVC is common in cables, plumbing hoses, and synthetic leather. Its fire resistance and durability make it indispensable in various industries.
These thermoplastics are essential building blocks for countless products, offering unique properties that cater to a range of functionalities and applications.
Polyester, nylon, and other common polymer-based plastics
Polyester and nylon are among the most widely used polymer-based plastics, valued for their versatility and strength. Polyester, commonly used in fabrics, beverage containers, and films, is prized for its durability, resistance to shrinkage, and ability to maintain its shape. Nylon, on the other hand, is a synthetic polymer frequently utilized in textiles, engineering plastics, and industrial applications due to its high tensile strength, elasticity, and resistance to wear and chemicals.
Other significant polymer-based plastics include polyethylene and polypropylene. Polyethylene, found in products like plastic bags and packaging, is lightweight, flexible, and moisture-resistant. Polypropylene, used in household goods, automotive components, and medical devices, combines toughness and chemical resistance with the ability to withstand high temperatures. Together, these materials form the backbone of modern manufacturing due to their adaptability to a wide variety of applications.
Natural polymers that can be used in plastic alternatives
Some natural polymers that can serve as sustainable alternatives to traditional plastics include starch, cellulose, and proteins like casein and soy. Starch, derived from crops such as corn or potatoes, can be processed into biodegradable materials suitable for packaging. Cellulose, a plant-based polymer, is used to create films and fibers with impressive strength and flexibility. Proteins like casein, sourced from milk, and soy can be transformed into bioplastics, offering strong and renewable options for various applications. These natural polymers are not only biodegradable but also help reduce our reliance on fossil fuels, making them a crucial part of the shift toward eco-friendly materials.
How do the physical properties of polymers affect plastic products?
Flexibility, strength, elasticity, and thermal stability are the properties of polymers, which are associated with the performance and design configuration of plastic products. For example, flexible low-density polymers are used for plastic bags and stretch wraps, whereas high-density, stronger polymers are suited for durable goods containers or automotive parts. The thermal characteristics of the polymer define its heat resistance and, therefore, suitability for temperature extreme applications. These characteristics enable manufacturers to customize plastic products for specific requirements, optimizing performance and efficiency.
Molecular weight and chain length: impact on plastic durability
Molecular weight and chain length are critical factors in determining the durability of plastics. Higher molecular weight translates to longer polymer chains, making the material stronger, more resistant to wear, and less prone to breaking under stress. This characteristic enhances properties such as tensile strength and impact resistance, which are vital for applications requiring robust and long-lasting materials, like automotive components or construction materials. Similarly, chain length influences a polymer’s crystallinity and flexibility—shorter chains tend to produce more brittle plastics, while longer chains improve elasticity and toughness. By controlling molecular weight and chain length during production, manufacturers can engineer plastics tailored to the demands of various industries, ensuring optimal durability and performance.
How additives modify polymer properties in plastic manufacturing
Additives play a crucial role in modifying and enhancing the properties of polymers to meet diverse application needs. Common types of additives include plasticizers, stabilizers, fillers, and flame retardants. Plasticizers increase flexibility and reduce brittleness by lowering the polymer’s glass transition temperature. Stabilizers improve resistance to heat, light, or oxygen degradation, extending the material’s lifespan. Fillers, such as calcium carbonate or glass fibers, enhance strength, stiffness, and reduce production costs, while flame retardants delay the spread of fire, improving safety in applications like electronics or construction. These additives allow manufacturers to customize plastic properties for specific uses, balancing functionality, durability, and cost-efficiency.
Why different polymers create plastics with unique characteristics
Different polymers create plastics with unique characteristics because each polymer has a specific molecular structure that determines its properties. For example, polyethylene has a simple structure that makes it flexible and lightweight, while polycarbonate is rigid and impact-resistant due to its complex arrangement. Factors like chain length, branching, and intermolecular forces significantly influence behavior, such as durability, heat resistance, or flexibility. By selecting or modifying polymers, manufacturers tailor plastics for precise functions, ranging from food packaging to aerospace components.
What is the difference between synthetic polymers and plastic?
Synthetic polymers are the building blocks of plastic, but not all synthetic polymers are classified as plastics. A synthetic polymer is a large molecule made from repeating units, synthesized through chemical processes. Plastics, on the other hand, are a subset of synthetic polymers designed for versatility, durability, and moldability. While synthetic polymers can include materials like nylon, polyester, and silicone, plastics specifically refer to polymers like polyethylene or PVC that can be shaped and used in countless applications.
When is a synthetic polymer considered a plastic?
A synthetic polymer is classified as a plastic if it can be shaped and retains that shape permanently. Usually, Changes are made to a material during its synthesis, such as softening, thermal strengthening, and increasing resistance to high temperatures. In general, plastics are defined as materials that can be mechanically processed and cut into specific geometric shapes through injection molding, extrusion, or thermoplasty.
Non-plastic applications of synthetic polymers
Synthetic polymers play an essential role in various industries apart from their use in plastics. One significant application is in textiles, where fibers like nylon, polyester, and spandex are derived from synthetic polymers to create durable, lightweight, and elastic materials. Another prominent use is in healthcare, with biocompatible polymers such as polyvinyl alcohol (PVA) and polylactic acid (PLA) being utilized for medical devices, drug delivery systems, and surgical sutures. Additionally, synthetic polymers find applications in adhesives, coatings, and sealants, offering functionality like water resistance, adhesion, and flexibility. Importantly, these materials are also used in electronics for creating semiconductors, insulating layers, and flexible displays, further showcasing the versatility of synthetic polymers beyond their molding capabilities.
The specific properties that make certain polymers ideal for plastic production
From my research, certain polymers are ideal for plastic production due to their unique combination of properties. High durability and strength allow plastics to withstand wear and tear, making them essential for a variety of applications. Their lightweight nature is another key factor, as it enhances portability and efficiency, especially in industries like automotive and aerospace. Additionally, polymers are versatile because they can be tailored to exhibit flexibility or rigidity, depending on the desired use. They also boast excellent resistance to chemicals, moisture, and corrosion, ensuring longevity. These properties, combined with cost-effectiveness and ease of production, make synthetic polymers indispensable in creating high-performance plastics.
How does understanding the polymer structure help with plastic recycling?

Understanding the polymer structure is crucial for effective plastic recycling as it determines how plastics can be processed and reformed. Different polymers have unique chemical and physical properties, such as melting points and molecular bonds, which dictate the recycling methods required. For instance, thermoplastics can be melted and remolded, making them more suitable for recycling, whereas thermosetting plastics are harder to recycle due to their cross-linked structure. Identifying the polymer type aids in sorting plastics accurately, ensuring efficient recycling and reducing contamination in the recycled material. This knowledge ultimately helps in creating a more sustainable recycling process.
Identifying different polymer types for effective plastic recycling
Identifying polymer types accurately is essential for efficient plastic recycling. Several methods are commonly used, combining practicality and reliability. First, the Resin Identification Code (RIC), found on most plastic products, serves as a basic guide. This numerical code ranges from 1 to 7 and indicates the type of polymer, such as PET (#1) or HDPE (#2), simplifying the sorting process.
Second, density testing can help differentiate plastics. By placing a sample in a liquid of known density, recyclers can observe whether the material floats or sinks to determine its type. For example, polypropylene (PP) floats in water, while polyvinyl chloride (PVC) sinks.
Finally, infrared spectroscopy offers a precise, advanced technique for analyzing plastics. Technologies like Fourier-transform infrared (FTIR) spectroscopy allow recyclers to identify polymer structures based on their unique spectral fingerprints. This method is particularly effective for distinguishing plastics with similar appearances.
Combining these methods ensures a more effective sorting process, reducing contamination and enhancing the quality of recycled materials.
Challenges in recycling different polymer-based plastics
Recycling polymer-based plastics presents a variety of challenges due to their diverse properties and applications. One major issue is contamination, as plastics often come mixed with food residues, adhesives, or non-recyclable materials, which complicates the recycling process and can degrade the quality of the recycled output. Another hurdle lies in sorting; many plastics are visually similar but differ significantly in their chemical composition, making accurate identification crucial yet resource-intensive.
Furthermore, multi-layered or composite plastics pose unique difficulties. These materials, such as packaging films made from layers of polyethylene and aluminum, are hard to separate during recycling, limiting their usability. The degradation of polymer quality during multiple recycling cycles also reduces the structural integrity of recycled products, creating limitations for their reuse. Additionally, the lack of standardization in plastic manufacturing globally exacerbates the challenge of creating scalable recycling processes.
Economic factors add to these challenges, as the cost of collecting, sorting, and processing plastics frequently outweighs their market value, reducing the incentives for recycling. Addressing these issues requires technological innovations, better design for recyclability, and stronger legislation to promote circular economies.
Innovations in polymer design for more recyclable plastic products
The improvement of polymer design remains one of the plastic recycling technologies’ greatest unresolved problems. One study focuses on developing new polymers like polydiketoenamine (PDK) that can be infinitely recycled without loss of quality. Other researchers are applying enhanced chemical recycling techniques to molecularly disassemble the plastic so it can be reformed into high-quality materials. There is also a growing interest in adding bio-based polymers with additives that are easier to break down and reform into new materials. These advancements will help reduce contamination and the decline in the quality of materials during recycling. For these solutions to be effective worldwide, there needs to be integration of different stakeholders in the industry, such as the producers, academics, and lawmakers to ensure that the solutions are aligned towards sustainability.
Are there environmental concerns specific to polymer-based plastics?

Yes, polymer-based plastics pose several environmental concerns. Their durability and resistance to degradation mean they persist in ecosystems for decades, contributing to pollution in landfills, oceans, and waterways. Microplastics, which result from the breakdown of larger plastic items, have infiltrated food chains, posing risks to wildlife and human health. Additionally, the production of plastics relies heavily on fossil fuels, leading to substantial greenhouse gas emissions. Inefficient recycling processes and contamination further exacerbate the issue, making proper disposal and innovative recycling techniques essential to mitigate their environmental impact.
Plastic waste persistence related to polymer molecular structure
From what I’ve learned, the persistence of plastic waste is directly linked to the molecular structure of polymers. Polymers are made up of long chains of repeating molecules, which are designed to be highly durable and resistant to environmental breakdown. This strength, while beneficial for product longevity, makes it incredibly difficult for natural processes to decompose them. Factors such as tightly bonded carbon-carbon backbones and the lack of functional groups that attract degradative enzymes mean that these plastics can remain intact for decades or even centuries. This inherent stability is a key reason why plastic waste is so persistent in the environment, and it emphasizes the urgent need for biodegradable alternatives and advanced recycling technologies.
Biodegradable polymers: a solution to plastic pollution?
Biodegradable polymers offer a promising avenue to mitigate plastic pollution by breaking down into natural elements through microbial activity under specific environmental conditions. Unlike conventional plastics, these polymers are designed with functional groups that facilitate enzymatic degradation, enabling quicker decomposition. For example, polylactic acid (PLA) and polyhydroxyalkanoates (PHA) are two widely researched biodegradable polymers, derived from renewable biomass like corn starch or sugarcane. However, the efficiency of their degradation largely depends on the surrounding conditions, such as temperature, moisture, and microbial presence, which are not always present in natural environments or landfills. Despite these limitations, the use of biodegradable polymers, combined with proper disposal systems and industrial composting facilities, has the potential to significantly reduce the environmental impact of plastic waste. Though not a standalone solution, they represent a critical component of a multifaceted approach to combating the growing global issue of plastic pollution.
How petroleum-based polymers contribute to plastic’s environmental impact
Petroleum-based polymers pose a significant environmental challenge due to their composition and lifecycle. These plastics are derived from non-renewable fossil fuels, contributing to greenhouse gas emissions during production. Once discarded, they degrade extremely slowly, often taking hundreds of years to break down, leading to persistent pollution in ecosystems. Additionally, their widespread usage results in microplastic accumulation in oceans and soil, harming wildlife and entering the food chain. Recycling rates for petroleum-based plastics remain low, as many are not economically viable to process, exacerbating the issue. Addressing these concerns requires reducing dependence on fossil-fuel-based plastics and promoting alternatives alongside robust recycling and waste management strategies.
References
Frequently Asked Questions (FAQ)
Q: What does it mean when we say plastics are polymers?
A: When we say plastics are polymers, we mean that plastics are a specific type of polymer. Polymers are usually comprised of a long chain of smaller molecules called monomers. Plastics are known as polymers because they are made up of these long chains.
Q: How are plastics and polymers related?
A: Plastics and polymers are closely related because plastics are a specific type of polymer. The term polymer refers to substances made of long chains of repeating units. Plastics, being polymers, are often synthetic and used to create objects of various shapes and utilities.
Q: Are all plastics made from petroleum?
A: Many plastics are made from petroleum, as it is a common source of the carbon and hydrogen atoms needed to form the polymer molecules. However, there are also naturally occurring polymers and bio-based plastics derived from other natural or synthetic sources.
Q: What are addition polymers and condensation polymers?
A: Addition polymers and condensation polymers are two main types of synthetic polymers. Addition polymers form the polymer by adding monomers together without losing any atoms, while condensation polymers release a small molecule, such as water, when monomers join together. Both types can be used to make plastics.
Q: Can plastics be molded into different shapes?
A: Yes, plastics can be molded into various shapes, which is one of their most valuable properties. The ability to mold plastics into objects of various forms, such as plastic bottles, plastic windows, and more, makes them highly versatile in industrial and consumer applications.
Q: What is a plastic resin?
A: A plastic resin is the raw material used to create plastic products. It consists of polymer molecules that can be molded into specific shapes. Plastic resins are the starting point for manufacturing a wide array of plastic items.
Q: Are there naturally occurring polymers?
A: Yes, there are naturally occurring polymers found in nature. Examples include cellulose in plants, proteins in animals, and natural rubber. These are distinct from synthetic polymers, which are man-made and include most plastics.
Q: How do synthetic polymers include plastics?
A: Synthetic polymers include plastics as they are engineered in labs through chemical processes that link monomers together to form long chains. These synthetic polymers are then molded into plastic products used in everyday life.
Q: What is the environmental impact of the amount of plastic used?
A: The large amount of plastic used globally has a significant environmental impact, contributing to pollution and waste. Efforts to recycle and develop new plastics that are more sustainable or biodegradable are ongoing to mitigate this issue.



